- What We Do
- Agriculture and Food Security
- Democracy, Human Rights and Governance
- Economic Growth and Trade
- Education
- Environment and Global Climate Change
- Gender Equality and Women's Empowerment
- Global Health
- Humanitarian Assistance
- Transformation at USAID
- Water and Sanitation
- Working in Crises and Conflict
- U.S. Global Development Lab
Speeches Shim

Lives are saved by timely access to quality-assured, affordable, effective and safe pharmaceutical products and services that ensure their appropriate use. Medicines treat tuberculosis, preeclampsia, and diabetes; vaccines prevent the spread of measles and other infectious diseases; diagnostic testing detects disease and reduces the overuse of antibiotics; insecticide-treated bed nets protect against malaria; and condoms limit the spread of HIV. Without essential medicines and other health technologies, and pharmaceutical systems that regulate, supply, and promote their appropriate use, the health needs of a population cannot be met.
Strong pharmaceutical systems are necessary for high-quality health care, particularly for the most-vulnerable populations. Many poor families must pay for basic health products that are a major source of waste and are frequently mismanaged or administered and used in the wrong way. It is estimated that half of all medicines are prescribed, dispensed, sold, or taken inappropriately, and many are of poor quality, all of which accelerates development and spread of drug resistance.
Pharmaceutical systems are particularly vulnerable to corruption because of the high value of medical products. All parts of the supply chain can be compromised by weak pharmaceutical systems, including delayed selection of essential medicines; calculation of quantities to purchase; ability of hospitals and pharmacies to purchase medicines; and warehousing and distribution of medical products..
For more than 20 years, USAID has led efforts to improve pharmaceutical systems in developing countries with programs in pharmaceutical management, including supply chain management and medicines quality assurance systems strengthening. These efforts have encompassed the development of innovative approaches and tools (including open-source applications) and addressed Agency disease programs (e.g., malaria, TB, child health) and cross-cutting (e.g., medicine safety/pharmacovigilance) issues.
Pharmaceutical systems strengthening is a key element in USAID’s Vision for Health Systems Strengthening 2015–2019. The Agency’s priority objectives in this area are to:
- Strengthen supply chain components to ensure the uninterrupted supply of quality-assured health commodities, including creating a supportive environment for commodity security.
- Strengthen regulatory capacity to protect the public from substandard and falsified products, and pharmaceutical sector governance to promote transparency and accountability through laws, regulations, policies, and standard operating procedures.
- Increase and enhance human and institutional capacity to manage pharmaceutical systems and services, including promoting evidence-based use of medications, assuring therapeutic efficacy, protecting patient safety, and slowing the emergence and spread of antimicrobial resistance.
A strong pharmaceutical system has established leadership and governance; effective regulation; robust financing for products and their management; a well-trained workforce and well-run organizations (from national institutions to local drug shops); reliable product and patient information; medical products and technologies available when and where needed; and services that promote rational use and protect patient safety.
The Agency conceptualizes this systems view in the following graphic:
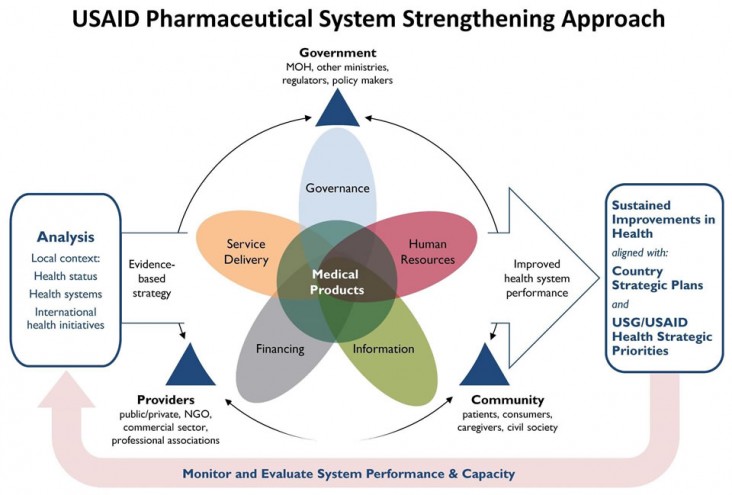
Learn more about our work in pharmaceutical systems strengthening:
New Tool to Measure Strength of Pharmaceutical Systems
USAID’s Medicines, Technologies, and Pharmaceutical Services program [PDF, 3.2MB]
USAID’s Promoting the Quality of Medicines program
USAID’s Global Health Supply Chain program
USAID’s System for Improved Access to Pharmaceuticals and Services program (2011-2018)

Comment
Make a general inquiry or suggest an improvement.